Cancer is characterized by uncontrolled growth of cells that invade and destroy adjacent tissues or spread to other locations in the body via lymph or blood. Cancer cells have the ability to penetrate and infiltrate surrounding normal tissues through metastasis, which is a complex cascade of events involving migration of cancer cells from the original site to other parts of the body usually lungs, liver, brain and bones through bloodstream or lymphatic system. It is reported that one of the critical steps necessary for cancer metastasis is tumor angiogenesis. Angiogenesis is a natural physiological process of growth of new capillary blood vessels from pre-existing vessels. Tumors secrete various growth factors such as basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), etc., for inducing blood vessel growth (angiogenesis). It is reported that cancerous cells stop producing the anti-VEGF enzyme protein kinase G (PKG) that apparently limits beta-catenin (a cancer promoting gene) in normal cells which solicits angiogenesis (Medical College of Georgia at Augusta University; EurekAlert). Since angiogenesis is also required for the spread of a tumor (i.e., metastasis), anti-angiogenic therapy is one of the most promising strategies for cancer treatment.
Like its parent compound, Tetrahydrocurcumin (THC) has shown both anti-proliferation and anti-angiogenesis effect (Yoysungnoen et al., 2008). More importantly, THC showed higher potent anti-angiogenesis effect than curcumin, which may be due to its higher antioxidant activity than curcumin.
THC was found to express anti-angiogenic potential without any cytotoxic affects on HepG2 cells even at the highest dose tested. It has suggested that anti-angiogenic properties of curcumin and THC represent a common potential mechanism for their anticancer actions (Yoysungnoen et al., 2008).
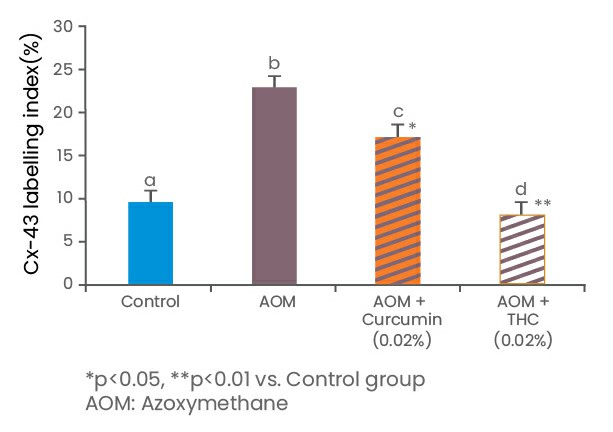
In a study on the development of preneoplastic aberrant crypt foci (ACF) induced by 1,2-dimethylhydrazine in colons of mice, it was found that THC was more active than curcumin in the inhibition of ACF development and cell proliferation (Kim et al., 1998). In a subsequent study undertaken by Sabinsa in co-operation with Rutgers, State University of New Jersey, tertrahydrocurcuminoids (THCs) were found to be more effective than curcumin in preventing azoxymethane-induced colon cancer. The results pointed out to the disruption of intercellular communication of crypt cells by THCs by reducing the levels of connexin-43 protein, an important molecule of gap junction.
All these evidences strengthen the promising role of THCs in the management of cancer.
Tetrahydrocurcumin (THC) is more effective than curcumin in preventing azoxymethane (AOM)-induced colon carcinogenesis
The study was conducted to investigate chemopreventive effects and underlying molecular mechanisms of dietary administration of curcumin and THC in AOM-induced colon carcinogenesis in mice. Mice were randomly divided into five groups of five animals each, four groups were administered with AOM at a dose of 5 mg/kg via an intraperitoneal (i.p.) injection twice a week for 2 weeks and then fed with diet containing curcumin (0.005 and 0.02%) or THC (0.005 and 0.02%).
The remaining group received injection of saline, fed with standard AIN-76 diet. It was observed that dietary administration of both curcumin and THC reduced aberrant crypt foci and polyps formation. The THC showed more significant inhibitory effect than curcumin. Further, results from western blot analysis and immune histochemistry staining showed that dietary curcumin and THC exhibited anti-inflammatory activity by decreasing the levels of inducible nitric oxide synthase (iNOS) and cycoloxygenase-2 (COX-2).
(Adapted from Lai et al., 2011)
These results conclude that dietary supplementation of curcumin and THC significantly reduced the level of connexin-43, an important protein molecule of gap junctions, impede the intercellular communication of crypt cells. There was no noticeable side effect or toxicity caused by dietary curcumin and THC treatment in rats (Lai et al., 2011).
THC, a major metabolite of curcumin, induced autophagic cell death through coordinative modulation of PI3K/Akt-mTOR and MAPK signaling pathways in human leukemia HL-60 cells
The present study reports that treatment with THC induces autophagic cell death in human cell lines (HL-60; promyelocytic leukemia cells) by increasing autophage marker acidic vascular organelle (AVO) formation. Human leukemia cell lines (HL-60) were grown in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, streptomycin 100 mg/mL and penicillin 100 U/mL. Inhibition of cell proliferation by curcumin or THC was measured by 3-(4,5-dimethylthiazole-2-yl)-2,5-biphenyl tetrazolium bromide (MTT) assay. It was observed from the results that the THC significantly down-regulated phosphatidylinositol-3-kinase/protein kinase B and mitogen activated protein kinase signaling including decreased phosphorylation of mammalian target of rapamycin, glycogen synthase kinase 3b and p70 ribosomal protein S6 kinase. The author of the study suggests that the use of THC as a new anticancer agent for human leukemia should be pursued further because of its prominent effect and its new anticancer mechanism of inducing autophagy (Wu et al., 2011).
