Curcumin the parent compound of tetrahydrocurcumin (THC) is a strong renal protective compound and much of its activity is related to reducing the inflammation in the renal tissue. The THC has also shown potential as a renal protective agent. Studies have shown that this activity partly arises from its antioxidant activity. THC ameliorates the oxidative stress induced renal injury in mice (Akiyama et al., 2000).
The renal protective effect of THC was studied in male ddY mice along with curcumin. Ferric nitrilotriacetate (Fe-NTA) was used to induce oxidative renal injury in the experimental animals before treatment with curcumin and THC. Fe-NTA increases the amount of free radical-associated modified molecules as assessed by lipid peroxidation products, aldehyde-modified proteins and a variety of modified DNA bases such as 8-hydroxy-2′-deoxyguanosine (8-OHdG) as early as 3hours of treatment with Fe-NTA. Therefore, oxidative suppression in the kidney was monitored by the formation of thiobarbituric acid reactive substance (TBARS), 4-hydroxy-2-nonenal (HNE)-modified proteins, 8-OHdG, etc., (Okada et al., 2001).
THC significantly suppressed the increase in lipid peroxidation and oxidative modification induced by Fe-NTA more effectively than curcumin. THC showed strong inhibitory effect against TBARS, HNE protein and 8-OHdG levels, suppression of the activity of glutathione peroxidase and phase II detoxification enzymes such as NADPH: quinone reductase (NADPH:QR) and GST (Okada et al., 2001).
Song et al. (2015) investigated the molecular mechanisms associated with the effects of THC on cisplatin-induced nephrotoxicity using in vitro cell culture and in vivo animal models. The authors used renal tubular cells (LLC-PK1) to determine the protective effect of THC against cisplatin-induced oxidative damage. Cisplatin treatment significantly decreased cell viability to about 50% compared to that of untreated control cells, whereas pretreatment with THC markedly restored cell viability in a dose-dependent manner. No cytotoxic effects of THC were observed at the treatment doses and these doses effectively protected cisplatin-induced cell damage.
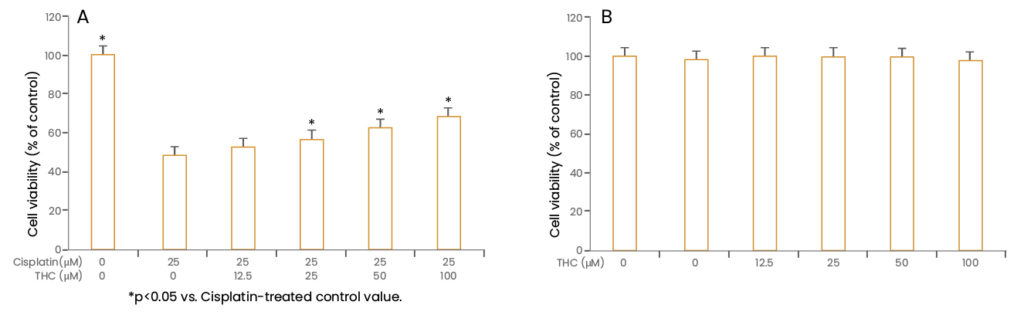
Effect of THC on cisplatin-induced nephrotoxicity in LLC-PK1 cells: (A) Dose-dependent protective effect of THC against cisplatin-induced nephrotoxicity in cells, (B) No cytotoxic effects of THC were observed at the treatment doses and these doses effectively protected cisplatin-induced cell damage. (Adapted from Song et al., 2015)
Next, the effect of THC on cisplatin-induced oxidative renal damage was examined in rats. Tetrahydrocurcumin treatment did not ameliorate cisplatin-induced body weight reduction and kidney weight increase. However, co-treatment with THC recovered the renal functional parameters, decreased creatinine clearance in cisplatin-treated rats. It was concluded that kidney cell damage induced by cisplatin was significantly inhibited by THC treatment. In addition, the renal dysfunction of cisplatin-treated rats was markedly ameliorated by THC administration. The renoprotective effect of THC was associated with the caspase-dependent anti-inflammatory pathway. Taken together, these results demonstrate the renoprotective effect of THC in cisplatin-treated rats and, therefore, its use can be considered to prevent kidney damage during or after chemotherapy.
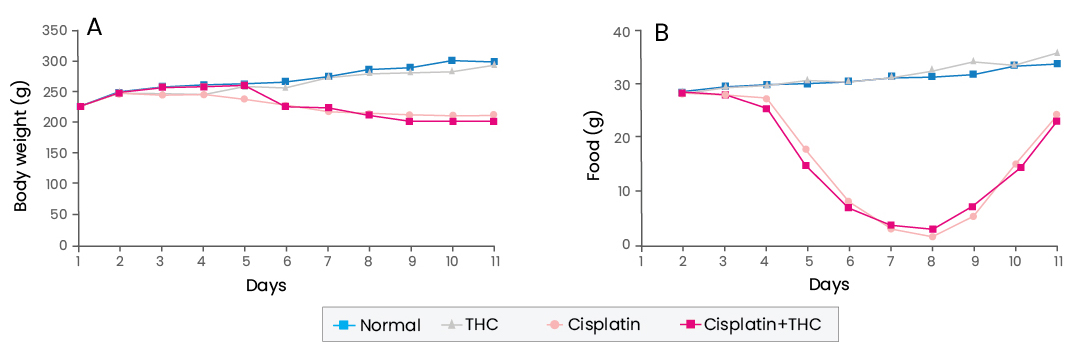
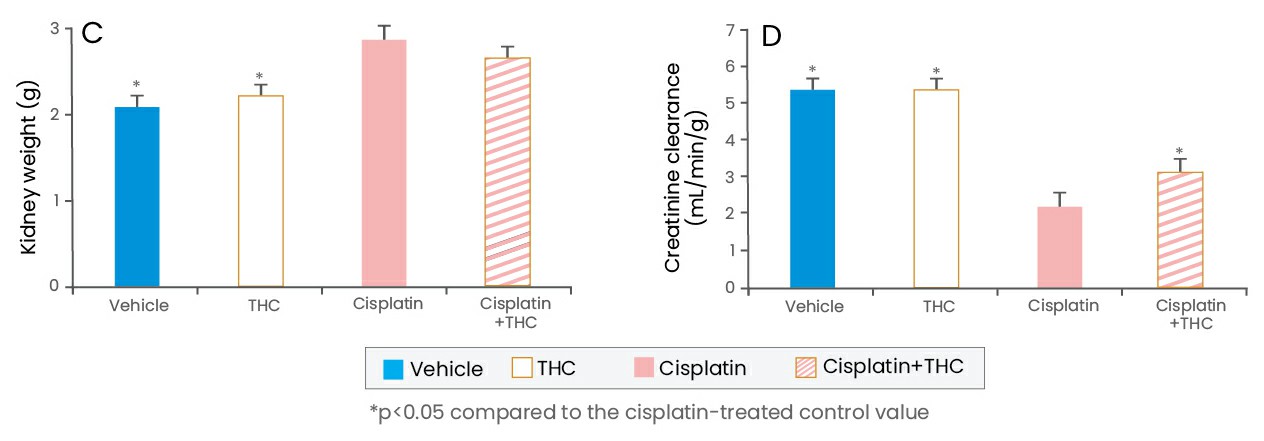
(Adapted from Song et al., 2015)
In a recent study Park et al. (2018) used LLC-PK1 cells to determine the protective effect and mechanism of THC against FK506-induced renal damage. It was reported that FK506 treatment (50 μM) reduced the cell viability by 55% compared to that of the control. The reduction in cell viability was ameliorated by THC co-treatment in a dose-dependent manner and observed upto 88% cell viability restoration after co-treatment with THC at a dose of 12 μM. In addition, THC showed antioxidant effect by restoration of GSH and anti-apoptotic effect by inhibiting the activation of caspase-3 and caspase-9. Thus, THC may act as an adjuvant therapy to reduce adverse effects of FK506 in the kidney.
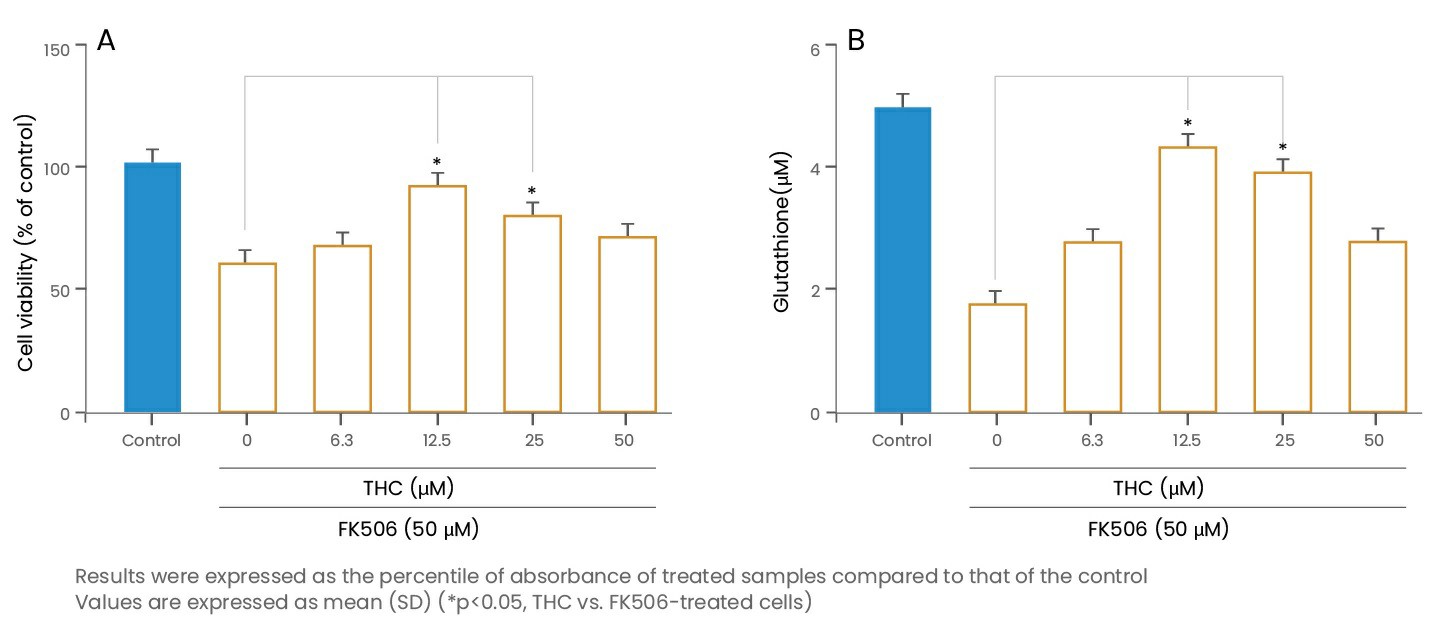
(Adapted from Park et al., 2018)
More recently, to investigate the beneficial renal effect of dietary THC, Lau et al. (2018) administered 1% THC to a well-established 5/6 nephrectomized CKD rats, which exhibit mass reduction of nephrons. The effect of THC on proteinuria, fibrosis, and inflammation was evaluated. It was reported that dietary THC treatment restored levels of the antioxidant (scavenging) enzymes metalloenzyme copper-zinc superoxide dismutase (CuZn SOD) and glutathione peroxidase (GPX-1), including iNOS that were suppressed in the remnant kidney from CKD rats. In addition, it was shown that apoptosis and fibrosis were increased in the remnant kidney from CKD rats as indicated by elevated caspase-3 and α-smooth muscle actin (αSM-actin), and these markers were decreased with THC diet. Tetrahydrocurcumin treatment-associated improvements on the markers of oxidative stress, apoptosis, and fibrosis in kidney lysates are illustrated in the below figures.
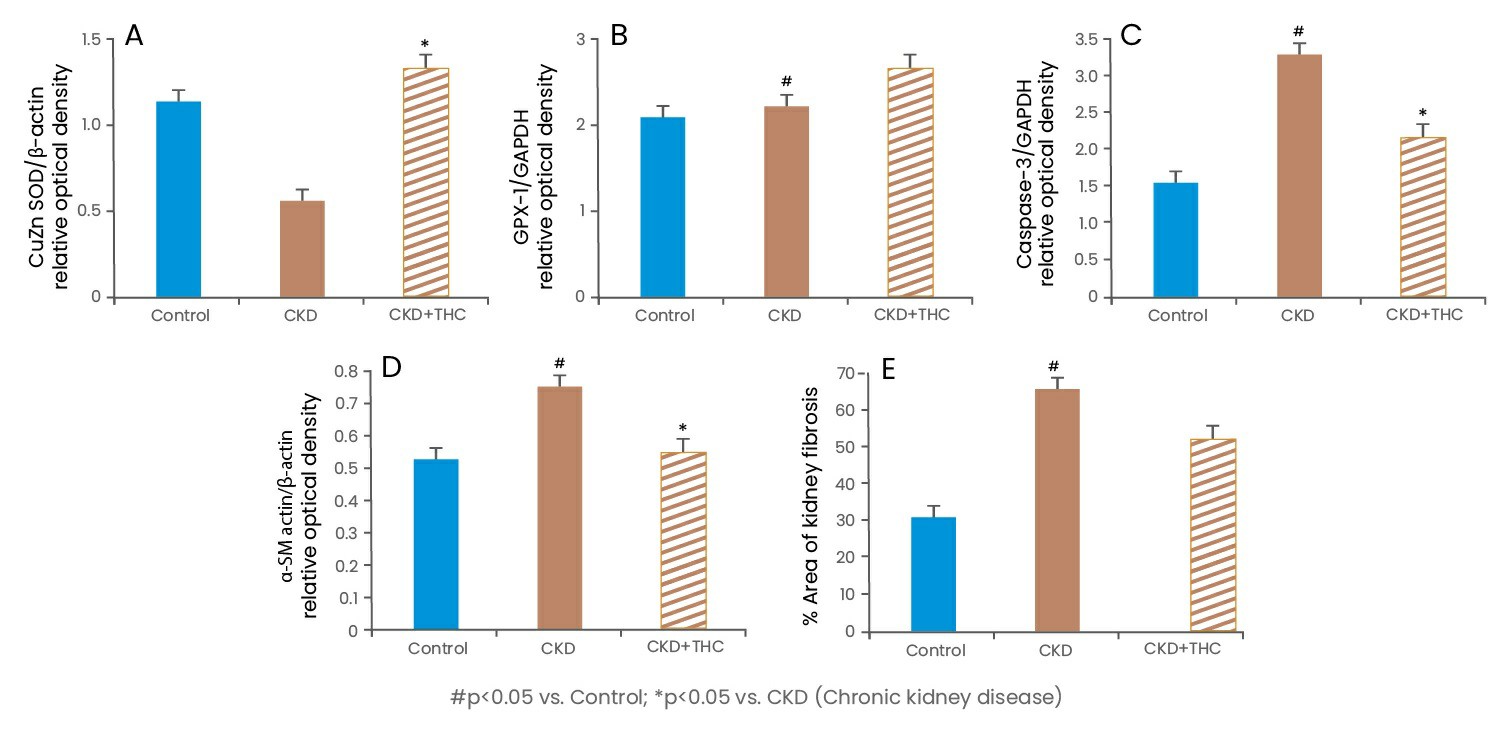
THC diet improved the protein markers of oxidative stress and kidney fibrosis. The antioxidant (scavenging) proteins: (A) Copper-zinc superoxide dismutase (CuZn SOD) and (B) Glutathione peroxidase (GPX-1) were decreased in CKD and levels were restored with dietary THC therapy. The apoptosis marker (C) Caspase-3 and the fibrosis marker (D) alpha-Smooth muscle actin (a-SM actin) were increased in the remnant kidney from CKD rats and were decreased with THC therapy. (E) Kidney fibrosis was decreased ~20% with THC therapy (Adapted from Lau et al., 2018)
